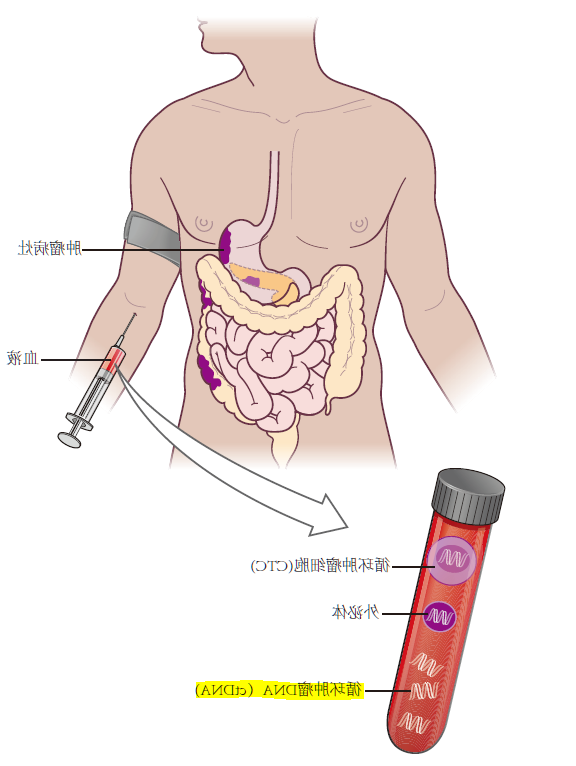
如今,免疫治疗和靶向治疗已经成为多种晚期实体瘤的首选治疗方法。然而,随着疾病的进展,需要实时监测治疗效果[1]。近年来,液体活检的广泛应用揭示了治疗过程中不断循环癌肿DNA(ctDNA)的动向變化与的方法反映迟钝增进涉及到,如果相对影象学,ctDNA就可以更早地判断的方法反映迟钝者[2,3]。
国内的专家学者在ctDNA高通量测序临床实践专家共识[4]中提出:
·ctDNA NGS 加测已被国内外专家共识或指南建议作为多种晚期恶性肿瘤组织基因检测的替代方式。
YunYing 允英出品
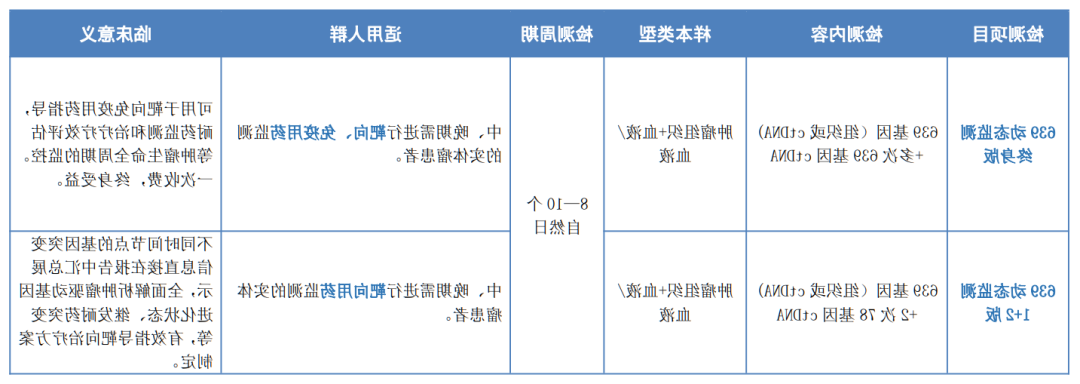
☆☆ 639动态图片监测系统 ☆☆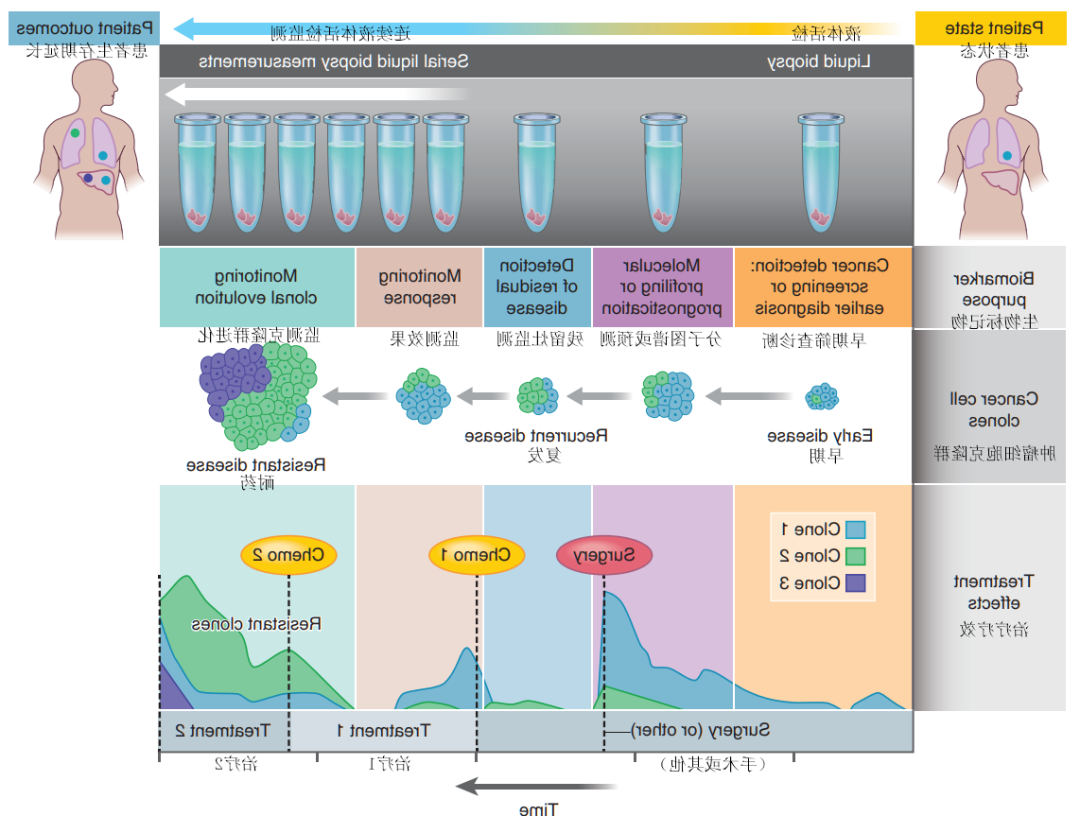
639日常动态数据监测 → 临床意义----评估靶向/免疫治疗疗效
▷ 抗体排查点调控剂的疗效鉴定: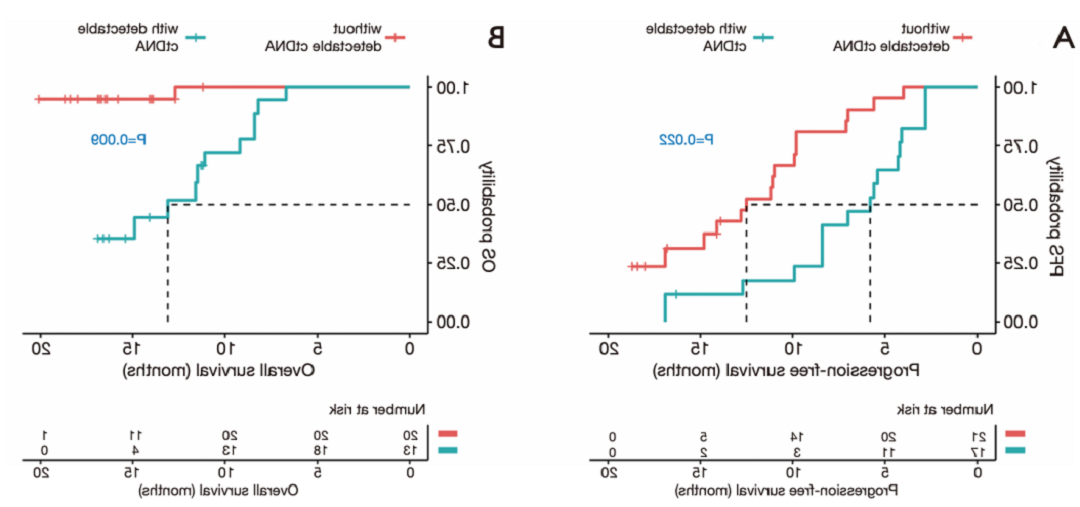
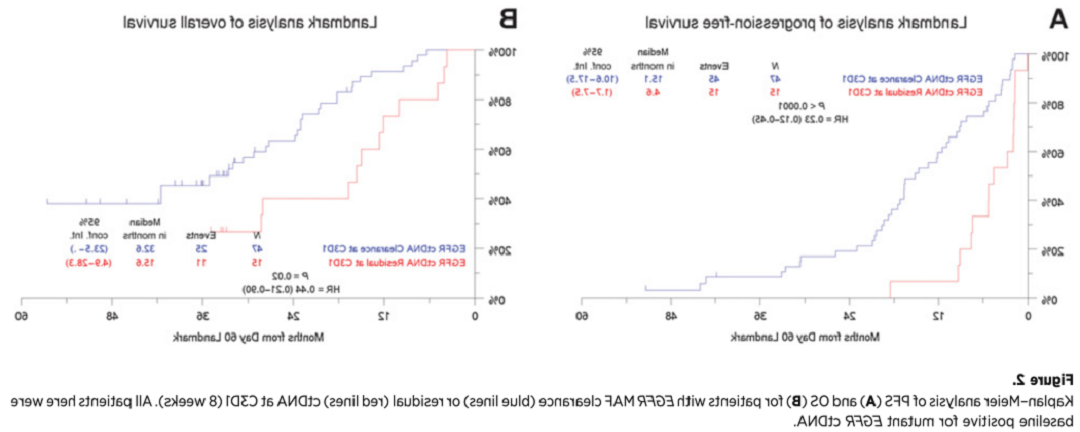
与组织学检测相比,ctDNA检测具有无创或微创,可反复取材,收集、同时能克服肿瘤空间异质性,可相对全面地实时反映患者的肿瘤分子特征等。
2021年,国际肺癌研究协会共识(IASLC)指出[10],ctDNA可作为初诊NSCLC患者基因分型的有效工具,也可作为诊断生物标志物评估和监测靶向治疗疗效的首选策略(其中血浆优先)。同时有研究显示[11],在转移性NSCLC中,ctDNA在线检测较仅采用了安排活检的女性可扩大48%的检测率。
获得性耐药是影响肿瘤精准治疗疗效的重要因素,也是抗肿瘤药物临床应用全程管理面临的最大挑战。例如在肺癌中:
·接受一代/二代EGFR-TKI药物治疗的晚期NSCLC患者发生耐药后,若检出T790M突变则可以换用奥希替尼进行治疗。对伴有EGFR突变的肺腺癌患者进行ctDNA动态监测,可对肿瘤的克隆演化进行早期评估,并可能在耐药相关临床表现出现之前开始针对性的干预。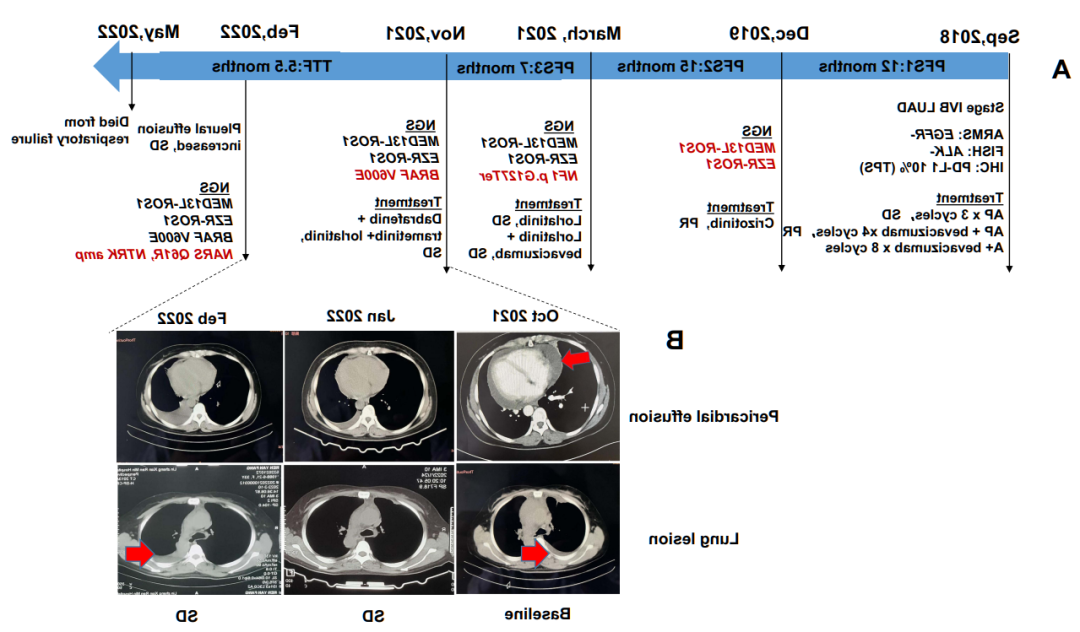

1、Yang Ching-Yao,Yang James Chih-Hsin,Yang Pan-Chyr,Precision Management of Advanced Non-Small Cell Lung Cancer.[J] .Annu Rev Med, 2020, 71: 117-136.
2、Jia Qingzhu,Chiu Luting,Wu Shuangxiu et al. Tracking Neoantigens by Personalized Circulating Tumor DNA Sequencing during Checkpoint Blockade Immunotherapy in Non-Small Cell Lung Cancer.[J] .Adv Sci (Weinh), 2020, 7: 1903410.
3、Zhang Qu,Luo Jia,Wu Song et al. Prognostic and Predictive Impact of Circulating Tumor DNA in Patients with Advanced Cancers Treated with Immune Checkpoint Blockade.[J] .Cancer Discov, 2020, 10: 1842-1853.
4、ctDNA高通量测序临床实践专家共识(2022年版)
5、Eroglu Z, Krinshpun S, Kalashnikova E, et al. Circulating tumor DNA-based molecular residual disease detection for treatment monitoring in advanced melanoma patients [published online ahead of print, 2023 Mar 4]. Cancer. 2023;10.1002/cncr.34716.
6、Ren S , Chen J , Xu X ,et al.Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous non-small-cell lung cancer (CameL-sq): a phase 3 trial[J]. 2021.
7、Lee Jenny H,Long Georgina V,Menzies Alexander M et al. Association Between Circulating Tumor DNA and Pseudoprogression in Patients With Metastatic Melanoma Treated With Anti-Programmed Cell Death 1 Antibodies.[J] .JAMA Oncol, 2018, 4: 717-721.
8、Ma Shenglin,Shi Meiqi,Chen Xueqin et al. The prognostic value of longitudinal circulating tumor DNA profiling during osimertinib treatment.[J] .Transl Lung Cancer Res, 2021, 10: 326-339.
9、Mack Philip C,Miao Jieling,Redman Mary W et al. Circulating Tumor DNA Kinetics Predict Progression-Free and Overall Survival in EGFR TKI-Treated Patients with EGFR-Mutant NSCLC (SWOG S1403).[J] .Clin Cancer Res, 2022, 28: 3752-3760.
10、Rolfo Christian,Mack Philip,Scagliotti Giorgio V et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer.[J] .J Thorac Oncol, 2021, 16: 1647-1662.
11、Leighl Natasha B,Page Ray D,Raymond Victoria M et al. Clinical Utility of Comprehensive Cell-free DNA Analysis to Identify Genomic Biomarkers in Patients with Newly Diagnosed Metastatic Non-small Cell Lung Cancer.[J] .Clin Cancer Res, 2019, 25: 4691-4700.
12、Li Dan,Liu Jiayin,Zhang Xue et al. ROS1 Combined Lorlatinib, Dabrafenib, and Trametinib Treatment for -Rearranged Advanced Non-Small-Cell Lung Cancer with a Lorlatinib-Induced V600E Mutation: A Case Report.[J] .Cancer Manag Res, 2022, 14: 3175-3179.
13、Hellmann Matthew D,Nabet Barzin Y,Rizvi Hira et al. Circulating Tumor DNA Analysis to Assess Risk of Progression after Long-term Response to PD-(L)1 Blockade in NSCLC.[J] .Clin Cancer Res, 2020, 26: 2849-2858.
14、2021.ESMO.1740P

杏彩体育旗舰厅 成立至今,顺利通过由国家卫健委临检中心、国家病理质控中心、美国病理学会等机构组织的室间质评。检验能力获得了美国病理学会PT认证,肿瘤核心基因检测试剂盒通过欧盟CE认证,获得国家药品监督管理局(NMPA)注册批准,且生产管理质量体系获国际ISO13485认证。目前允英已累计为近三十万例肿瘤患者提供精准检测服务,为国内肿瘤精准医疗领域开拓者。同时允英秉承“严谨、高效”的理念,坚守分子诊断技术创新与应用探索,为肿瘤精准医疗与伴随诊断领域的茁壮发展贡献力量。